Welcome to Suckville, A Week in Quarantine
We were told to wait in a curtained alcove in Memorial Hermann’s imaging center. Outside, a catatonic elderly woman lay on a gurney clutching a teddy bear while two orderlies adjusted her IV and joked about their weekend plans–oblivious to the hooded man with a scythe standing nearby.
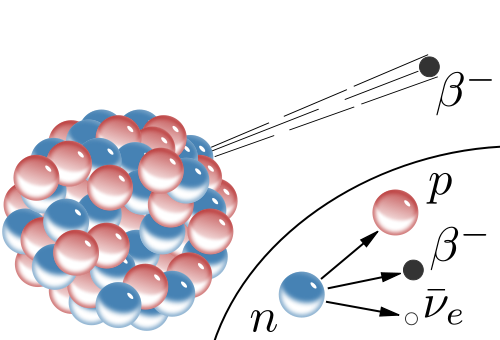
The radiologist flipped open the curtain and entered carrying a lead jar. Looking over her shoulder, she whispered, “we should wait until she leaves.” The jar held a single radioactive pill known as I-131. The I for Iodine; the 131 for the atomic weight of the isotope. The stable weight of Iodine is 127, which means that each atom of this isotope has four extra neutrons. This excess creates instability. In the case of I-131, nature eventually fixes the imbalance by transforming the extra neutrons into protons. But this conversion also releases high-energy electrons that burst out of the nucleus like so many fireworks.

These fast-traveling globs of negativity called Beta particles are the basis for Beta radiation, a radiation that wrecks havoc on that which it encounters–chemical bonds, etc. So, it’s a good idea to steer clear of Beta Radiation as a rule. And yet, here she was, about to ingest a pill filled with 0.8 micrograms (100mC) of this isotope.
We heard the laughing voices recede down the hall; the woman was gone. The green-gloved radiologist began unscrewing the jar. Inside sat a single pill in a plastic vial. She lifted it out and handed it to her. “Pour this pill into your mouth. Don’t touch it.” Steph gave a wide-eyed look and obeyed.
“You should leave the hospital immediately and avoid contact with anyone on the way home.” Fortunately the hospital was only five minutes from our Heights home.
They speak of radioactive isotopes in terms of “half-lives,” which is a statement of averages. Because these nuclear changes occur according to a probability distribution and not a precise, predictable moment, the half-life of an isotope represents the average time it takes for half of its atoms to transmutate back into a stable form. Curiously, the converted form of I-131 is no longer Iodine at all; it’s Xeon.
The half-life of I-131 is eight days, which is short compared to, say, Uranium-238 with its 4.4 BILLION year half-life. An eight day half-life means that eight days after taking the pill, half of the remaining I-131 will have been radiated. Eight days after that, half of that half will have been radiated, and so on. But–and this is important–the vast majority of the bad stuff gets excreted out of the body through urine, sweat, and other bodily fluids long before it decays. They estimate that by day four, upwards of 99% will have been excreted. The upshot: don’t share bathrooms. The toilet will be the most radioactive spot in the house for a month.
 So why all the radioactivity? Thyroid cancer has a really handy characteristic. Thyroid cells are unique in that they alone eat iodine. They need iodine to produce their hormones, tetraiodothyronine (T4) and triiodothyrodine (T3). Since Steph had her entire thyroid removed two months ago, she’s been enjoying a steady diet of synthetic T4 replacement pills called Synthroid (T4 transforms into T3 naturally; T3 is crucial to your metabolic system). T4 takes a long time to wash out of the system. But T3 disappears very quickly. In anticipation of this Radioactive Iodine therapy (RAI), they first switched her to synthetic T3 (Cytomel) and eventually took her off all medication. They wanted the remaining thyroid cells to be fully active (“hungry”). Unfortunately this meant she was devoid of these energy-giving hormones. It’s called being Hypothyroid and it completely sucks.
So why all the radioactivity? Thyroid cancer has a really handy characteristic. Thyroid cells are unique in that they alone eat iodine. They need iodine to produce their hormones, tetraiodothyronine (T4) and triiodothyrodine (T3). Since Steph had her entire thyroid removed two months ago, she’s been enjoying a steady diet of synthetic T4 replacement pills called Synthroid (T4 transforms into T3 naturally; T3 is crucial to your metabolic system). T4 takes a long time to wash out of the system. But T3 disappears very quickly. In anticipation of this Radioactive Iodine therapy (RAI), they first switched her to synthetic T3 (Cytomel) and eventually took her off all medication. They wanted the remaining thyroid cells to be fully active (“hungry”). Unfortunately this meant she was devoid of these energy-giving hormones. It’s called being Hypothyroid and it completely sucks.
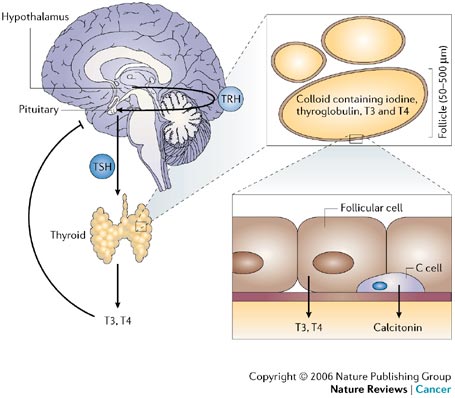
RAI treatment capitalizes on all of this to target any remaining thyroid cells that are also cancerous. Once a thyroid cell consumes I-131 its fate is sealed. Fortunately its Beta Radiation has an extremely limited range (2mm), i.e, nearby cells are rarely affected. The other advantage to this treatment is that obliterates all remaining thyroid cells, even the good ones. In the future a simple blood test will be used to check for thyroid activity. New cells would be bad (=cancer).
Ironically, I-131 not only kills thyroid cancer, it can cause it. I-131 is produced during the nuclear fission of atomic bombs and the fallout from reactors. The incidence of thyroid cancer rose significantly in places like post-war Japan and the midwestern United States after the cold war nuclear tests. Here’s a good article about that: http://www.atsdr.cdc.gov/csem/csem.asp?csem=23&po=4. This all comes back to the importance of isolating patients receiving RAI therapy.
Throughout this week, she has exhibited most of the typical I-131 side effects: headaches, neck pain, metal taste, and stomach aches. The biggest worry was about potential damage to her salivary glands, which unfortunately also consume iodine. Thus far, there’s been no sign of that.
10-14 days after RAI, radiologists typically perform a Whole Body Scan (WBS) to see where the iodine was consumed. What they’re actually seeing is the gamma radiation from the isotope’s breakdown since the decay of I-131 releases both Beta and Gamma radiation. Gamma is much less harmful and easy to detect with gamma-scanning machines. Most of the iodine will be sitting in the kidneys waiting for excretion. The rest should only be seen in the thyroid bed in the neck. In all likelihood, Steph’s scan will show a cancer-free body. That’s what the doctors are telling us. That’s what my gut says too.
Her WBS is scheduled for Wednesday. Expect a clean bill of health and the end of the cancer episode.
This is how our week went…







3 Comments
daniellereich
Love love love love love love love to Steph. That was a scary read. Hugs.
Cathy
That is so f*&%#d up!
Sean
Hoping to see both of you doing something else soon!